PILLARS OF LIFE
Before going crazy spreading weird creatures like it's the Cambrian Explosion, let's first take a quick look at how life goes from Primordial Soup to some primate on a computer reading mah blog...
WARNING, THIS TOPIC IS VERY EXTENSIVE !
Time counting scale reading:
1Ky = 1 Thousand years (Kiloyears) ; 1Kya = 1 Thousand years ago.
1My = 1 Million years ; 1Mya = 1 Million years ago.
1By = 1 Billion years ; 1Bya = 1 Billion years ago.
No, not there yet buddy...
Lets come back to our planet...
Mine in the case, during the course of this blog, every biology stuff related to Earth-like planets will be centered in the planet Paart, from the Vol System. Not at random that Vol can mean VOLUNTEER.
Being a pretty complete system, and around a pretty old star, we can study and make observations about the entire evolution of simple star systems and life within them.
As said in previous post, Vol is a star old enough that Paart could start life from ZERO 4 TIMES before Vol turns the system uninhabitable. That's exactly what we are going to do along the blog, explore 4 possible scenarios for life to develop before a catastrophe ruin everything.
Just to catch back from where we left, here is the Vol System:
And here are the planets to scale:
I have never defined Paart's body data, so lets do that...
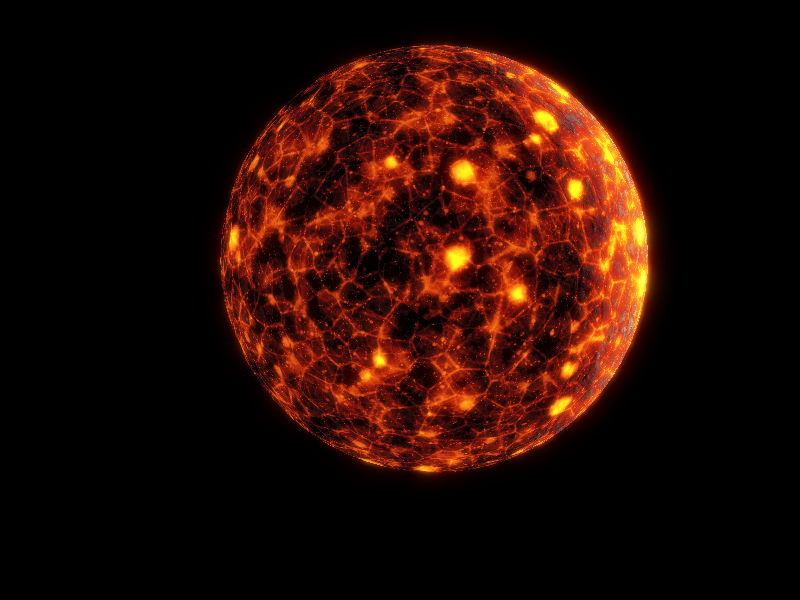
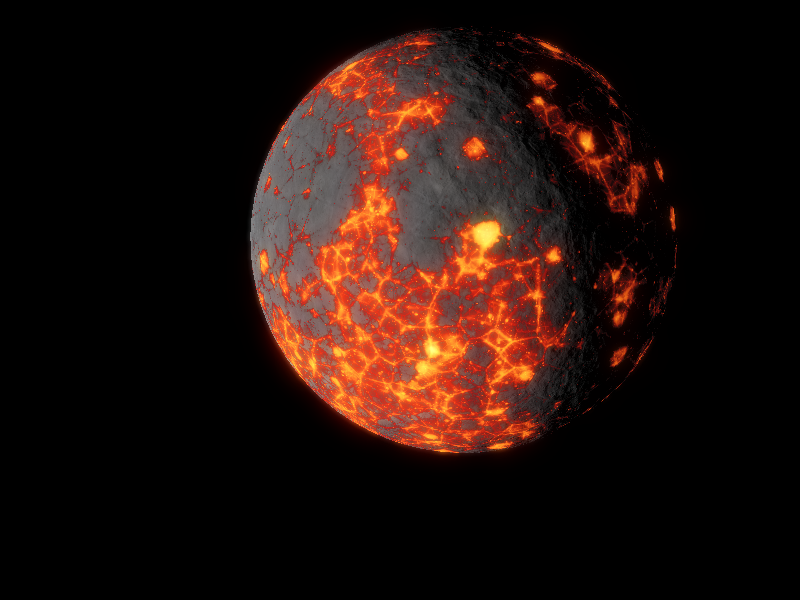
Planet image is a young-Venus made by me
SYSTEM BASIC ORBITAL DATA:
VEEK - 0,31 AU
HOOL - 0,58 AU
PAART - 1,11 AU
COMET - 2,15 AU
SEEY - 4,20 AU
LAHAART - 8,26 AU
I can figure out the other system's planets in future topics about planet types...
During the formation of the Vol System - pretty much like our Solar System and other young systems we are able to observe - there was chaos, rocks and vapor wandered around the young star, starting to condense and form the primordial blobs of rock and gas that gave origin to at least ~100 planets in the first 250My of existence, some get ejected out the star system by gravitational resonance of major bodies or get fused together, other may be cracked out in asteroid belts and ejected in very elliptical orbits that take WHOLE EONS to complete a lap around Vol (spoiler alert, there will one of those soon).
Pretty much every rocky planet goes through a phase called
HADEAN, after Hades, the greek underworld god, often described as a hellish landscape, is the chaos phase during planet formation, I think there is no much need exactly tell how it happens, just a bunch of hot rocks crumpling around into bigger rocks, keep in mind from last post, that it takes usually ~500My for a planet similar to Earth in size/mass to cool down enough.
Bellow, some images made by me...
By the time Paart is cooled down, at age 550My, Vol as young star shines with roughly 25% less energy than Main Sequence, which means that for now, that its habitable zone lies between 1,29AU and 0,43AU, in our best scenarios, Paart is currently on the outer edge of the HZ.
Also means that Vol is a smaller star by 10%, being currently 1,19 million kilometers wide.
What takes us to the...
FAINT YOUNG SUN VOL 'PARADOX'
What we currently know about young stars?
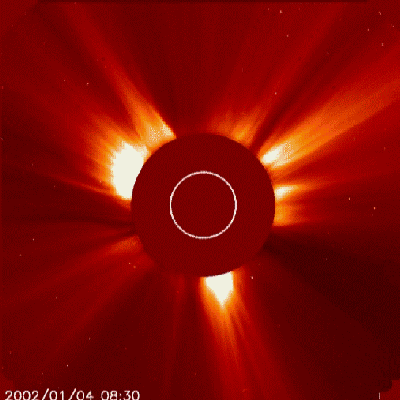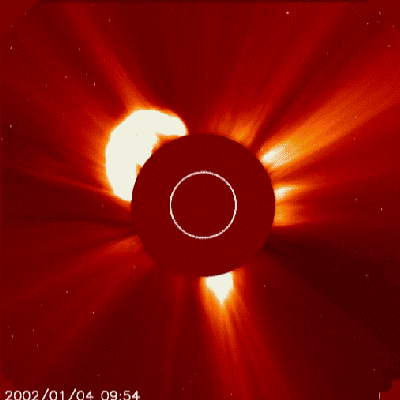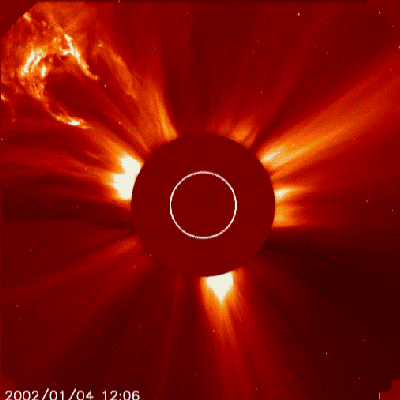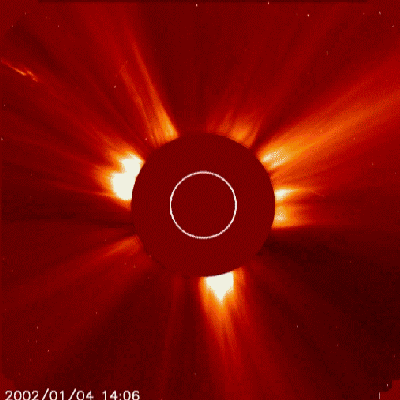
They are very active, believed that the early life of stars are as Flare Stars, when the magnetic field from nuclear fusion carries away lots of atmospheric material from the star and throws it away to outer space in bursts of plasma, X-ray and alpha particles (Helium nuclei), known as Coronal Mass Ejections.
pretty much like this...
Today, a few of those are bright and powerful enough to do some damage to Earth, and when it happens, it shoots to any other direction but Earth. When the Sun was younger it happened more frequently, and of course it hit Earth a dozen, a hundred, a million times during 1 to 2 billion years...
The Earth is ~4,5By old, just count back ~2By and we found that Mars probably received a so powerful charge that it lost it's magnetic field and atmosphere (helped by asteroid impact in the northern hemisphere led to core cooling and thus, no volcanic activity to renew the soil or melt remaining ice).
SO WHAT?!... >:/
SO WHAT that this GIF never been so accurate lol
The atmosphere of the early Earth as we can read from rocks were pretty different, formed by Nitrogen gas and Carbon compounds, almost no Oxygen, which means NO OZONE.
With a more violent Sun, and no ozone, x-ray and ultra-violet can easily reach the surface of the planet, and heat it up, not only by being in the most energetic range of the Electromagnetic Spectrum, but by Photo-dissociation,
high-energy photons can easily break the chemical bounds in any compound, as the most abundant reactive one was the water in Earth's oceans, we have now a source of Hydrogen gas and mono-atomic Oxygen.
Which reacts with the Nitrogen gas creating Nitrous Oxide (N2O) which is ~300x more efficient in holding heat than Carbon Dioxide (CO2), also, Hydrogen gas reacts with mono-atomic Carbon to create Methane (CH4) which is ~30x more efficient than Carbon Dioxide, Hydrogen Cyanide (HCN), and Ammonia (NH3), along with Sulfur compounds.
During day-time this intense radiation bombardment would eventually also destroy these compounds, at night with a very active magnetic field due reaction to strong stellar wind and flares, free electrons can create ecumenical storms around the young Earth since there is not great continents that can stop winds and tsunamis, electrical storms can restore greenhouse gases broken during the day at night and maintain a reasonable balance of heat required for rain to fall down with those chemicals into the oceans...
"the entire world is now an ocean"
As Vol ages, radiation bombardment gets less frequent and eventually allows the "photo-greenhouse cycle" to be broken.
By the way, ~4Bya is possible that Venus beared with the same conditions as Earth, remember the young flare-Sun? Now remember some geology, gases get trapped in rocks, rocks tell us what kind of atmosphere Earth had at the time they formed, we can't yet study a rock from Venus, BUT, we now have some idea...
During the Hadean of Venus, as well as Earth's and any other rocky worlds, gas and rock vapour were free to circle the ball of flaming rocks that was Venus, those gases got trapped in its rocks, and through Photo-dissociation those gases can be released to the atmosphere, we know that Venus lacks free oxygen and oxygen gas, that makes up ozone, no ozone means free way to high-energy X-ray and UV light to release trapped gases from surface rocks. Venus may had oceans like Earth, even shallow ones would do the work, water combined with sulphur compounds makes acids, like sulphuric acid, which corrodes more rock, releasing more greenhouse gas.
Not being protected by an ozone layer means that when the greenhouse effect evaporates all its water to the higher atmosphere, it will be dissociated into Oxygen and Hydrogen gas, later combined with Carbon to make carbon dioxide, or be mostly wiped out to outer space, and maybe, captured by early Earth.
The now ionized toxic gas atmosphere of Venus is full of carbon dioxide and vitriol clouds by the same effect that saved Earth from being a snowball in it's early days.
Personally, I advise you to when making calculations about planetary distance to star, attempt not making it too close to its star, being at the HZ doesn't make it habitable, account this Flare-phase of your star to it, too close and photo-dissociation will turn you planet into a Venusian hell too fast, too far away and it won't happen at all and turn your planet into a radioactive desert like Mars, unless it's your intention from the start.
Attempting making similar compositions for your planet's crust as it is from Earth, changing some few percent can be game-changing already for biological and physical purposes.
By now, is utterly good that our planet Paat lies on the outer HZ.
Archean Earth by Norbert Toth on ArtStation
How our planet might look by age 640My...
Paving the way...
Here are some vids to help you understand how chemical soup turns into LIFE:
And some inspiring history of our planet:
The first life...
OKAY, the Coacervate (some times called Microsphere), is formed from naturally occurring fatty acids layers bent into a sphere, which isolates the inner room from the outer world. This coacervates allow new chemical reactions to occur inside them, but they're not alive YET.
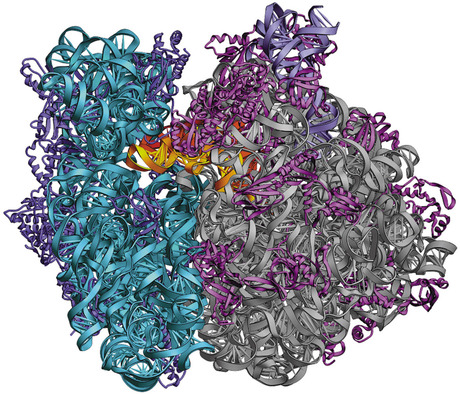
A ribosome
A ribosome is 'nothing more' than entangled molecules than replicate other molecules around.
Modern cells uses ribosomes as a jack-of-all-trades, need some protein? Ribosome can do it! Need some fatty acid? Ribosome can do it! Need some RNA? Ribosome can do it! Need a girlfriend? Ribosome CAN'T DO IT . _. <sigh>
You got the picture here... Oversimplifying, a coarcervate is dumb/primitive ribosome that is able to reproduce even tho not being alive, how?
Ever blowed a soap bubble in a way it split in two or more? If no I recommend you to test it yourself.
Not exactly like a soap bubble, but a coacervate is made of fatty acids bent into a sphere by hydrostatic equilibrium and water polarity, if you have/absorb/inject too much content into a coacervate it will naturally split into two, that's why it was initially thought to be alive, it grew, reproduced and had movement.
Coacervates are nature's blank canvas for life, or like test tubes for a chemist, they are places you can make reactions and test chemical systems.
Chemical reactions need energy to occur, it can be already stored in the chemical bounds of the reagents, or provided by an outer source, like heat from a lab stove or sea chimney, or light from a lamp or the Sun.
Earth's oceans had turned dark brown/purple with the primordial soup, created with the chemicals from the atmosphere that rained for millions of years. Dark colors absorb light, which means that our oceans had turned into a warm "rotten" fluid that goes as far you can see the horizon. Earth's atmosphere was full of ions created by the radioactive bombardment of the young-Sun, as well ones created during thunderstorms, ions need counterparts to stabilize, so once those ions reach the water, they're great starters for chemical reactions near the surface.
At the bottom of the ocean, we also have ions along with volatile substances that are essential in supplying coarcevates with material for making their stuff, cooking new molecules inside them.
Molecules that overreacted to the environment of the coacervate, eventually destroyed it, which means that it can't continue to react, the ones that helped the overall structure to keep itself "alive" got reproduced along, as coacervates fuse during collision or heating those isolated chemical substances get to react together and if doesn't destroy the new environment it is allowed to continue existing, and yes, that's a parallel with plasmid transfer that some bacteria and archaea actually do for survival.
I'd like to say that Earth's early days were like "Sandbox Mode" for pre-life things, you got a very energetic environment when all you need are some dozen calories to exist. Those things went crazy testing the periodic table around and mixing recipes, until we have something complex as ribosome being born, and oh boy, yeeeee boyyyy, what do you think it will happen if a pretty advanced coacervate swallows one that is able to build its own stuff instead of just selectively absorbing pre-made parts???
PROTO-CELL! By absorbing the right kind of 'pseudo ribosomes' you can have your own "organelles".
Early Earth's atmosphere and seas was full of what??? Simple stuff, hydrogen, carbon dioxide, acetates, formates, methanol and methylamines.
once most of sunlight is blocked by the soup itself, energy can be drawn from those compounds reactions, this creates Methane, and then you have your first methanogens, develop a way of preserving this recipe, like chunks of simple RNA, and they later can evolve into the first archaea that are far better in doing that.
Note: Methane is a greenhouse gas, and as such, it raises water vapour into the atmosphere, water later dissociated by UV and turned in some small amount of Ozone and hydrogen gas, of course, depending of its concentration it can be harmful or not to the biosphere bellow.
Remember I said the ocean is now purple? Purple is on the energetic side of the visible spectrum along with blue, as well ultraviolet. By nature this thing is ridiculously dangerous for our proto-cells and our archaea. Ultraviolet and X-ray broke the water and rock apart for millions of years, that's why we now have hydrogen and a handful of greenhouse gases in the air in the first place. We (first life) want to keep that away.
As the waters beared with all types of 'creatures', mostly reflecting the UV like a hot potato through the water. We can assume that some unprepared little ones obviously got dissolved when hit by ultraviolet, and some reacted to it, hydrogen and carbon are required to make methane inside of what we now can call a CELL, there is also water along with other chemicals. When ultraviolet breaks water inside the cell it releases hydrogen and oxygen, we can also assume it may break some carbon dioxide, when those react at some proportions you have either alcohol, which kills the cell from inside, or SUGAR, which can be broken later for ENERGY.
Hey little cell, you just figured out Photosynthesis!
Now you just need to do that again, and again, maybe create a substance that reacts better with UV and increase your food production, you now can be either red, black or green.
WAAAAIIIIT!!!
OH NO!
I think we just released some oxygen gas into the atmosphere, and that stuff is toxic for you anaerobic comrades. Sorry, it was a pleasure to... To... Swim around with ya?
The Phylogenetic Tree of Life
Even tho your story doesn't directly involve a swimming microscopic being, remember The Phylogenetic Tree of Life, that small change in the environment eons ago will give some microscopic life certain apparatus to be inherited by its offspring over the phylogenetic tree. 'Cause, it doesn't make sense any alien species having some special ability if their planet's fauna, their phylogenetic relatives doesn't have it, even to some extent.
History of early life doesn't have to end here (yet), what if instead of conventional photosynthesis, they used the chlorine cycle? Or Helium hydrate as agent for reactions? Or still, leading to breathe hydrogen compounds (hydro breathers are a topic I actually used in one fiction story I wrote). Imagine the possibilities of biological warfare for the sea control before it evolve into moss made of styrofoam or PVC, even white blood of titanium oxide.
Whatever weird chemistry your aliens may have, it starts here, in a warm pool containing all sorts of chemicals and amino acids, set at the sun, in a warm world, under a strange sky...
- M.O. Valent, 03/05/2019